Lesson 3 - What is soil pH?

The pH of soil describes its acidity or alkalinity, terms that many gardeners have heard of, but perhaps don’t know exactly what they mean. Scientifically pH is a logarithmic scale based on the concentration of hydrogen ions present in solution. The scale runs from 0 to 14 with pH 7 being neutral, pH values lower than 7 indicating acidity, and values above 7 being alkaline. Soils are described as being increasingly ‘acid’ as the pH drops, and increasingly ‘alkaline’ as pH increases. Because pH is logarithmic, close values on the scale are ten times more acid or alkaline than the next value. For example, soil with pH 4 is 100 times more acid than a soil with pH 6.
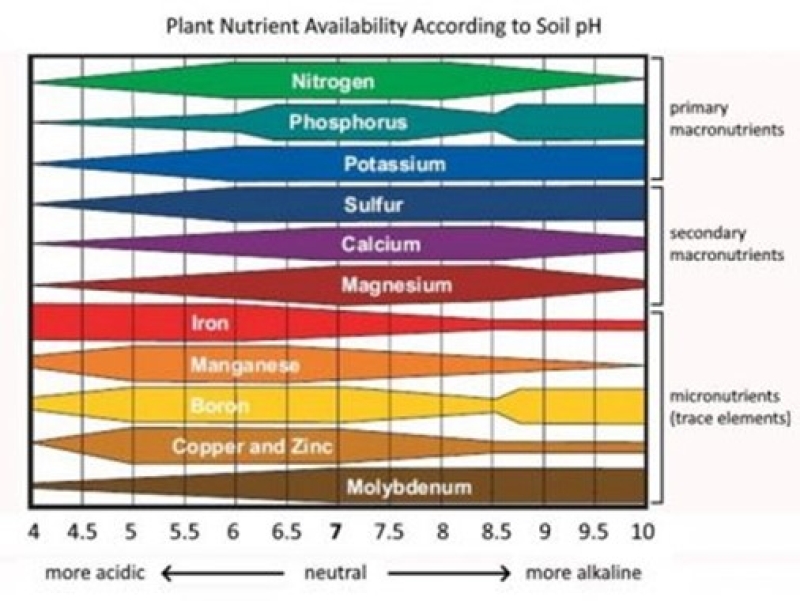
Soil pH is important because it changes the availability of nutrient elements to plants. Plants evolve in soil that has a specific pH range, and we sometimes describe them based on this as being ‘acid-loving’ (e.g. blueberries) or ‘lime-loving’ (asparagus). When plants are grown in conditions outside of their natural pH range, they will frequently show symptoms of nutrient deficiency or toxicity. For many plants, particularly vegetables, there is some flexibility in the soil pH in which they will grow successfully, but this is typically only to one, or perhaps two, units on the pH scale. Outside of this range their nutrient needs for successful growth will be unmet (deficiency) or in excess (toxicity). The diagram below shows this.
While not shown in the diagram, another specific problem associated with soil acidity is the increased solubility of aluminum (Al). While all soils contain Al, in acid soils it becomes more soluble in acid soils, becoming quite toxic to sensitive species.
Because of the effects of nutrient availability and toxicity, the best pH for growing food in most soils will be between 5.5 to 7.5. This value range is a little different if you are growing in containers with a potting mix that is based on organic materials such as pine bark, coir, composted sawdust and peat moss. In this situation the ideal pH range will be between 5 to 6.5.
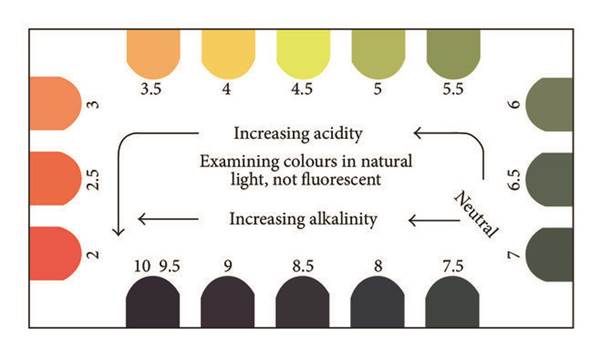
There are several ways you can test the pH of your garden soil. Makes sure you have a representative sample by collecting small amounts of soil from across the area (5-10 places) and mix these together, breaking up any clods or lumps. Always test your topsoil and subsoil separately. Perhaps the simplest method is a colourmetric test kit (see video). This method uses a tiny amount of fine, dry soil mixed to a paste with a few drops of indicator dye, which is then sprinkled with a powder that draws out the colour to make interpretation easier (Barium sulphate). After a few seconds a distinct colour of the sample is revealed which can be compared to a pH colour chart to determine the pH. These test kits are available through Diggers and most large garden centres.
Another method of testing soil pH is a small electronic hand meter, a device with an electrode at its base to measure pH. This method requires mixing up a slurry of a known amount of your soil sample with a volume of deionised water, generally a 1:5 ratio of each in terms of volume. The electrode of the meter is then placed into this slurry with the pH value then being displayed on the screen. These meters are more expensive than a colourmetric test kit but can be used repeatedly. Some are also able to measure soil salinity (electrical conductivity). Perhaps the most common pH testing devices available for purchase are soil probes with a digital screen displaying the pH after insertion into the soil. These are not recommended. They are notoriously unreliable and inaccurate, some recording pH as much as two pH units out.
The most common methods for adjusting the pH of soils are to raise it (make it more alkaline) or to lower it (make it more acidic). Increasing pH is done by adding a ground liming agent, the most common being agricultural lime (Calcium carbonate), builders lime (calcium hydroxide) or dolomitic lime (blend of calcium and magnesium carbonate). The amount needed to change the pH is dependent on the type of lime you use and your soil texture. Products like agricultural lime and builders lime have a much higher concentration of limestone present compared to dolomite, meaning they elevate pH more efficiently. Clay soils require much more lime compared to sandy soils (about 4 time the amount). A one- unit change in pH (raising from pH 5 to 6) in a loamy soil requires about 200 grams per square metre mixed in well with the top 100 mm of soil. A change in pH will take about 2 months if you are using builders’ lime, and up to 12 months for straight lime. Further testing is often needed, and generally some sort of annual application is required to avoid the drift towards acidity.
Lowering soil pH is more complex, particularly where pH values are greater than 8. If you have a soil pH less than pH 8 you can try adding organic matter like composts, manures or peat, although this can take years to be effective. A quicker and more accurate method is to use elemental sulphur, also called agricultural sulphur. When added to the soil, sulphur is converted to sulphuric acid, increasing hydrogen ions and lowering the pH. In a loamy soil you will need to add about 50 grams of sulphur per square metre (half of this for sands, double the amount for clays) to reduce the pH from 8 to 7. It will generally take around 6-8 weeks to be effective and will last for about a year. Iron sulphate can also be used to lower pH but requires much greater amounts than sulphur to be effective. As per raising pH, with soils that are naturally alkaline, annual additions of sulphur will be needed to maintain a lower pH. If your soil pH is greater than 8 it is very difficult to reduce and this may determine which crops you can grow successfully.